WLA VA:COVID19: Difference between revisions
Mceledon83 (talk | contribs) |
Mceledon83 (talk | contribs) |
||
| Line 190: | Line 190: | ||
* Commercial lab = ~ 3-4 business days | * Commercial lab = ~ 3-4 business days | ||
== | ==Treatment== | ||
* | * General: | ||
** | ** No specific treatment currently available | ||
* | ** Non-pharmaceutical interventions will be most important | ||
* | *** Spread Prevention | ||
** | *** Mitigation strategies | ||
* High-dose corticosteroids should be AVOIDED (due to progression of viral replication reported from prior coronaviruses; e.g. MERS, SARS) | |||
* Avoid nebulizers as they are generally ineffective may aerosolize virus | |||
** Albuterol with spacer is safer, though probably ineffective unless co-occuring reactive airway disease | |||
* Generally avoid BiPAP and high-flow nasal oxygen as these may increase viral spread | |||
** WHO cautiously states that high flow oxygen may be occasionally indicated. | |||
*Intubation: | |||
** High risk procedure for aeresolization | |||
*** Patient ideally in negative pressure room. Limit individuals in room to essential staff only. | |||
*** PPE for all in room: N95, gown, gloves, eye shield | |||
*** PPE for provider intubating: Consider PAPR, double glove, double gown | |||
*** BVM with viral filter | |||
* Use sufficient paralytics to prevent coughing gagging | |||
* Most experienced provider should perform intubation. | |||
* Ventilate using ARDSnet protocol (https://www.wikem.org/wiki/EBQ:ARDSnet_Trial) | |||
*Experimental/compassionate use treatments: | |||
** Remdesivir (IV) | |||
*** CDC does not recommend for or against any investigational therapies at this time | |||
*** Contact Gilead directly for use: compassionateaccess@gilead.com | |||
*** Background: novel antiviral nucleotide analog. Initially developed for Ebola and Marburg (has since been found to show activity against other single stranded RNA viruses such as RSV, Lassa fever virus, Nipah virus and the coronaviruses including MERS and SARS) | |||
**** 3 clinical trials across country (one is NIH adaptive trial) | |||
**** 2 other trials are investigational open-label trials testing different dosages for moderate or severely hospitalized patients | |||
** Ritonavir also being used but no data available. Same for chloroquine. | |||
==Decision To Hospitalize== | ==Decision To Hospitalize== | ||
Revision as of 03:18, 18 March 2020
Introduction
- Disease name = COVID-19
- Virus = SARS-CoV-2 (previously 2019-nCoV
Virology
- Coronaviruses are a common human pathogens (discovered in the1960s to cause the common cold).
- During epidemics, they are the cause of up to one-third of community-acquired upper respiratory tract infections in adults; may cause diarrhea in infants and children as well)
- SARS-CoV-2 is a novel coronavirus (a new strain not previously identified in humans)
- Likely primary source = bats
- A betacoronavirus in the same subgenus as the severe acute respiratory syndrome (SARS) virus
- Receptor-binding gene region is very similar to that of the SARS coronavirus (uses angiotensin-converting enzyme 2 [ACE2] for cell entry)
- Middle East respiratory syndrome (MERS) virus, another betacoronavirus, appears more distantly related
- Viral survival time of SARS-CoV-2:
- Stainless steel: persists for 3 hours (or longer)
- Underscores the importance of environmental cleaning / disinfection
- Cleaning gets rid of the proteins that would interfere with a disinfectants effectiveness
- Note: studied in a simulated lab environment. Lab virions not covered in protein and mucus and other things that would mimic real life and that could prolong survival
Basic Epidemiology/Infectivity Data
- Expected patient outcomes (from data so far):
- 80% have mild symptoms
- 15% have severe disease requiring hospitalisation
- 5% require mechanical ventilation
- Case fatality rate (CFR) = 2-4% (from Hubei data)
- SARS ~ 10%
- MERS ~ 35%
- Seasonal flu ~ 0.1-0.2%
- 1918 Pandemic Influenza ~ 2-3%
- R0 = 2.2 - 4.2
- Where R0 = expected number of secondary cases produced by a single typical infection in a susceptible population (basic reproductive rate)
- R0 for seasonal flu ~ 1.3
- R0 for pandemic flu ~ 1.5-1.8
- Incubation: 5 days (median); range of 2-14 days
- Serial interval duration = 7.5 days
- Serial interval refers to the time from illness onset in successive cases in a transmission chain
Clinical Findings
- At onset: fever, dry cough, myalgias, fatigue, shortness of breath
- Fever and cough start early, SOB noted about 9 days into illness
- Fever not present in all adults (less common in vulnerable populations)
- Less common: cough with sputum, sore throat, headache, congestion, GI symptoms
- Most common complications: pneumonia, ARDS (avg 8 days from onset, 20% of patients in China)
- Decompensation risk occurs during 2nd week of illness
- Risk factors: older adults, underlying conditions (lung disease, heart disease, diabetes)
- Children: milder disease (see Children)
- Pregnant patients: don’t appear to be at increased risk of infection or adverse outcomes (limited data - see Pregnant Women)
Laboratory Findings
- Lymphopenia most common in critically ill; mildly elevated ALT, AST; normal pro-calcitonin on admission
- Elevated d-dimer and severe lymphopenia are associated with increased mortality
- RT-PCR is currently test of choice for confirming cases
- Test kit availability is currently limited as of mid March
- Consider influenza/viral respiratory panel to identify alternative diagnoses
- Although co-infection is possible
Imaging
- XRay:
- Portable CXR preferred in PUI to prevent spread of infection
- May be normal in early disease
- Typical pattern is peripheral patchy ground glass opacities (GGO)
- More opacities correlates with worse disease
- GGOs may coalesce and appear as infiltrates
- CT:
- Many have normal imaging early on (so CDC DOES not recommend CT for diagnostic purposes at this time)
- CT (86%) more sensitive than CXR (59%) for detecting GGOs
- Radiopaedia COVID-19 Resources (https://radiopaedia.org/articles/covid-19)
- From the American College of Radiology (3/11/20): “Generally, the findings on chest imaging in COVID-19 are not specific, and overlap with other infections, including influenza, H1N1, SARS and MERS. Being in the midst of the current flu season with a much higher prevalence of influenza in the U.S. than COVID-19, further limits the specificity of CT.”
- Reinfection (after recovery from COVID19): unclear if possible
- Limited data. Unlikely to be reinfected shortly after but unknown about later on
General Prevention Recommendations
- Exercise general infection precautions
- Person-to-person transmission occurs with close contact (6 feet)
- Direct contact with mucous membranes or respiratory droplets
- Indirect: cough —> secretions left on surface —> 2nd person touches surface secretions and touches face & mucous membranes
- Avoid touching your face (try it, it’s not easy)
- Frequent Handwashing
- Alcohol based hand sanitizer
- Diligent hand wasing
- 20 seconds minimum
- Image shows commonly forgotten areas: thumb (ulnar aspect), fingertips, WRIST (Borrowed from WHO Hand Hygiene for Healthcare)
- Wear a mask if you develop respiratory symptoms (fever, cough, rhinorrhea, congestion) to prevent spread
- Person-to-person transmission occurs with close contact (6 feet)
- Avoid unnecessary travel
- Stay home if symptomatic
- Home care does not mean being out in the parks with other groups of people
- Contact your supervisor: due to expected HCW shortages, minor symptoms may be allowed to continue working with adequate PPE to prevent infection spread
Precautions For Healthcare Workers
PPE Bottom Line: Per CDC and LADPH (3.12.20)
- “Can collect specimens (e.g., nasopharyngeal swabs) for COVID-19 observing standard, contact, and droplet precautions including eye protection in a normal examination room with the door closed”
- No airborne isolation required (unless aerosol-generating procedure)
Transmission
- Simply walking into a room is NOT a recognized risk of transmission. Must make contact with respiratory droplet (directly or indirectly)
- Masks: MOST IMPORTANT utility is to put on the coughing individual
- Research clearly demonstrates it decreases shedding of infectious material in the environment
- This is more effective than HCWs wearing masks prophylactically to prevent catching the infection when not actually performing close contact patient care
- How long to shut a patient room down after a COVID patient is in there?
- It’s not about the risk of contracting the infection but about the ability to clean room safely without respiratory protection precautions by the cleaner
- 30-40 minutes usually sufficient (for most modern facilities) as long as no aerosol-generating procedure performed (longer, time not clearly stated at this time)
- Most modern rooms designed to have 12 air exchanges per hour
- Ventilation symptoms vary. So, older / fewer exchanges per hour => more time.
PPE Guidelines
- EVERY PATIENT CONTACT: Respiratory droplet precautions. Contact precautions also recommended but if gowns in short supply consider reserving for aerosol-generating procedures
- Droplet = surgical mask, eye protection
- Contact = gown and gloves
- Technique:
- Mask donning (often incorrectly done):
- Wash hands BEFORE touching mask
- Grip mask by loops/bands/ties only
- Coloured portion typically faces outward
- Mold / pinch the stiff edge to the shape of your nose
- Pull the bottom of the mask over your mouth AND chin
- Make sure you are up to date with fit testing
- Mask removal:
- Wash hands BEFORE touching mask
- Only make contact with the loops/bands/ties. DON’T TOUCH THE MASK ITSELF!
- Mask donning (often incorrectly done):
- For AEROSOL GENERATING procedures: airborne precautions (N95/PAPR)
- Due to higher risk of aerosolizing droplets-- infection itself doesn’t seem to be spread via airborne route)
- Aerosol generating procedures (avoid when possible)
- Bag-valve mask (BMV)
- CPAP/BiPAP
- Intubation
- Nebulizer administration (COMMONLY FORGOTTEN) - use MDI instead. E.g. 8-12 MDI puffs instead of albuterol 2.5-5mg INH.
- Bronchoscopy
- Chest PT
PPE Shortage/Limiting Usage Guidelines
In case of PPE shortage or in an attempt to save on PPE supplies, the following guidelines were approved by CDC 3/13/20
- Same respirator can be worn for multiple serial patient contacts (e.g. in between successive COVID/PUI (patients under investigation) without exchanging respirator. Therefore, in between each patient:
- No need to change mask or eye protection
- BUT need to change gown and gloves
- Respirator reuse possible? Higher risk because of having to touch the mask and either self-inoculate or transmit to another patient (e.g. wear it for a patient, then you remove, and then you put it back on)
- If you must do this because of limited supplies, don and doff properly and perform proper hand hygiene in between
- CDC / NIOSH will allow certain N95s to be used beyond manufacture-designated shelf life
- See list of appropriate models here (manufactured between 2003-2013)
- N95 Reuse? Probably okay to re-use same N95 during an 8 hour shift as long as no tears or visible contamination. Store facedown in labeled re-sealable bag/container.
- Based on non peer reviewed reports from Washington State
Healthcare Worker Monitoring
- Every HCW should be keeping a thermometer at home
- Self-monitor BID (and especially before work). Facilities should screen their HCW prior to shifts.
- If symptomatic, notify supervisor.
- If febrile, STAY HOME.
- If other symptoms, discuss with supervisor / clinical experts. Due to expected HCW shortages, minor symptoms may be allowed to continue working with adequate PPE to prevent infection spread
Isolation
- Persons diagnosed with COVID-19 are considered cleared after 14 days from symptom onset or 3 days after resolution of fever and improvement of other symptoms, whichever is longer.
- CDC: Reasonable to isolate patients with unexplained fever and respiratory symptoms (and no travel history) at this time
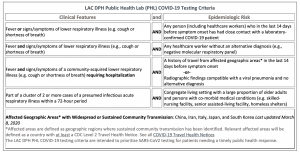
Testing
- LA County DPH checklist (http://publichealth.lacounty.gov/acd/ncorona2019/checklist.htm)
- Mild illness: DO NOT send for testing (increased risk of exposure to COVID-19)
- ER: DO NOT go unless hospital level of care is needed (increased exposure of other patients and staff)
- Testing can be done in ambulatory setting if absolutely needed (see precautions)
Guidelines: Epidemiologic Factors
- Persons (including HCW) within 14 days of travel (domestic/international), or
- Close contacts with lab-confirmed COVID19 patient within 14 days
Criteria For Sending Specimen to PHL
Criteria For Sending Specimen to Commercial Lab
- Patients with fever and cough/shortness of breath not requiring hospitalisation who have:
- History of travel from affected geographic areas (domestic / international) within 14 days of symptom onset
- Other exposure risk indicated by the patient’s history and clinical judgement (and no alternative diagnosis -- e.g. negative flu test)
Decision To Obtain Imaging
- CXR: no significant issues with contamination/disinfection
- CT: Temporarily out of commission after COVID19 patient in scanner
Clinical Sample Collection
- Best way to collect:
- Upper respiratory tract and lower tract specimens (if available).
- NP swabs
- Put both of them in the same tube and send for a single test
- For productive cough patients: can collect sputum to send for testing. CDC does NOT recommend inducing sputum (because aerosol generating)
- Upper respiratory tract and lower tract specimens (if available).
Testing: Turnaround Time
- LA County Public Health Lab (PHL) = ~ 2 business days
- Commercial lab = ~ 3-4 business days
Treatment
- General:
- No specific treatment currently available
- Non-pharmaceutical interventions will be most important
- Spread Prevention
- Mitigation strategies
- High-dose corticosteroids should be AVOIDED (due to progression of viral replication reported from prior coronaviruses; e.g. MERS, SARS)
- Avoid nebulizers as they are generally ineffective may aerosolize virus
- Albuterol with spacer is safer, though probably ineffective unless co-occuring reactive airway disease
- Generally avoid BiPAP and high-flow nasal oxygen as these may increase viral spread
- WHO cautiously states that high flow oxygen may be occasionally indicated.
- Intubation:
- High risk procedure for aeresolization
- Patient ideally in negative pressure room. Limit individuals in room to essential staff only.
- PPE for all in room: N95, gown, gloves, eye shield
- PPE for provider intubating: Consider PAPR, double glove, double gown
- BVM with viral filter
- High risk procedure for aeresolization
- Use sufficient paralytics to prevent coughing gagging
- Most experienced provider should perform intubation.
- Ventilate using ARDSnet protocol (https://www.wikem.org/wiki/EBQ:ARDSnet_Trial)
- Experimental/compassionate use treatments:
- Remdesivir (IV)
- CDC does not recommend for or against any investigational therapies at this time
- Contact Gilead directly for use: compassionateaccess@gilead.com
- Background: novel antiviral nucleotide analog. Initially developed for Ebola and Marburg (has since been found to show activity against other single stranded RNA viruses such as RSV, Lassa fever virus, Nipah virus and the coronaviruses including MERS and SARS)
- 3 clinical trials across country (one is NIH adaptive trial)
- 2 other trials are investigational open-label trials testing different dosages for moderate or severely hospitalized patients
- Ritonavir also being used but no data available. Same for chloroquine.
- Remdesivir (IV)
Decision To Hospitalize
- Mild symptoms may go home and self-isolate/quarantine
- Note: symptoms may worsen over 2nd week of illness
- Hospitalize: Respiratory distress/failure, multi-organ failure, rapid disease progression requiring escalating supportive care
- May consider discontinuation of hospital isolation when:
- Resolution of fever without anti-pyretic, resolution of symptoms, and negative COVID19 testing