Gout and pseudogout
Pathophysiology
- Primarily an illness of middle-aged males and elderly adults
- Gout in females usually occurs only after menopause
- Gout is most common form of inflammatory joint disease in men >40yr
- Presence of crystals does not exclude septic arthritis
Precipitants
- Trauma
- Surgery
- Medication (allopurinol, thiazide/loop diuretics, ASA)
- Alcohol consumption
- Meat/Seafood consumption
- Dehydration
- Lower body temperature
Clinical Features
- Joint pain may develop over period of hours
- Primarily involves first MTP, knee, ankle
Differential Diagnosis
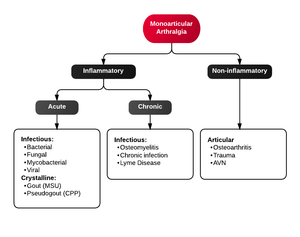
Monoarticular arthritis
- Acute osteoarthritis
- Avascular necrosis
- Crystal-induced (Gout, Pseudogout)
- Gonococcal arthritis, arthritis-dermatitis syndrome
- Nongonococcal septic arthritis
- Lyme disease
- Malignancy (metastases, osteochondroma, osteoid osteoma)
- Reactive poststreptococcal arthritis
- Trauma-induced arthritis
- Fracture
- Ligamentous injury
- Overuse
- Avascular necrosis
- Decompression sickness
- Spontaneous osteonecrosis
- Hemorrhagic (e.g. hemophilia, systemic anticoagulation
- Seronegative spondyloarthropathies (ankylosing spondylitis, IBD, psoriatic arthritis, reactive arthritis
- RA, SLE
- Sarcoidosis, amyloidosis
- Periarticular pathology
- Transient (Toxic) Synovitis (Hip)
- Slipped Capital Femoral Epiphysis (SCFE)
- Legg Calve Perthes Disease
Evaluation
- Synovial fluid aspiration
- Gout = Negatively birefringent, needle-shaped monosodium urate crystals
- Pseudogout = Positively birefringent, rhomboid-shaped, calcium pyrophosphate crystals
- Serum uric acid levels are not helpful (30% of patients with gout attack have normal levels)
- Uric acid during attacks can be low due to the precipitation of gout crystals
- High uric acid is >6.8
- ESR may be elevated
- No bacteria on Gram Stain
- Pseudogout: X-ray of joint space may show radiolucent calcium pyrophosphate crystals
Arthrocentesis of synoval fluid
| Synovium | Normal | Noninflammatory | Inflammatory | Septic |
| Clarity | Transparent | Transparent | Cloudy | Cloudy |
| Color | Clear | Yellow | Yellow | Yellow |
| WBC | <200 | <200-2000 | 200-50,000 |
>1,100 (prosthetic joint) >25,000; LR=2.9 >50,000; LR=7.7 >100,000; LR=28 |
| PMN | <25% | <25% | >50% |
>64% (prosthetic joint) >90% |
| Culture | Neg | Neg | Neg | >50% positive |
| Lactate | <5.6 mmol/L | <5.6 mmol/L | <5.6 mmol/L | >5.6 mmol/L |
| LDH | <250 | <250 | <250 | >250 |
| Crystals | None | None | Multiple or none | None |
- Viscosity of synovial fluid may actually be decreased in inflammatory or infectious etiologies, as hyaluronic acid concentrations decrease
- The presence of crystals does not rule out septic arthritis; however, the diagnosis is highly unlikely with synovial WBC < 50,000[1]
Management
Patients usually only require opioid and NSAID treatment in the ED with continued NSAID treatment as an oupatient. However any combination of the following treatments are acceptable[2]
All patients
- Hold diuretics
- Consider starting losartan to replace diuretic (has modest uricosuric effect)
- Alcohol and dietary counseling
- Continue uric acid-lowering agents if already on prophylactic regimen (do not start)
- Follow up with Primary Doctor or Rheumatology if having continued flares
NSAIDs
- Do not give to patients with renal insufficiency (use opioids instead)
- Substantial pain relief should occur within 2hr
- Options:
- Indomethacin 50mg po TID x3-5d, OR
- Naproxen 500mg po BID x3-7d, OR
- Ibuprofen 800mg PO TID x 3-5d
Other options
Colchicine
- Can be used as alternative agent to NSAIDs in patient with normal renal/hepatic function
- 1.2mg PO (load), followed by 0.6mg one hour later x 1 [3]
- May use to maximum of x3 doses, with more aggressive regimens totaling max dose up to 6mg[4]
- Wait at least x3 days before starting another round of colchicine therapy
- Renal impairment not absolute contraindication for acute flare but may consider dose reduction.
- Dose adjustments for CYP3A4 inhibitors (HARRT, Calcium Channel Blockers, fluconazole, erythromycin, clarithromycin)
- Colchicine should not be administered intravenously
Initial: 0.6-1.2mg, followed by 0.6 every 1-2 hours; some clinicians recommend a maximum of 3 doses; more aggressive approaches have recommended a maximum dose of up to 6mg. Wait at least 3 days before initiating another course of therapy
Steroids
- Prednisone 30 to 50mg initially, and gradually tapered over 10 days, results in clinical resolution without rebound pain or complications[5][6]
Glucocorticoid injection
- Even if gout has been diagnosed in the past, be cautious with glucocorticoid joint injection if the current clinical picture is uncertain since a septic joint can coexist with gout and a steroid injection would then worsen the patient's clinical status.
Other Therapies
- Anakinra
- Disease-modifying agent; Interleukin-1 Receptor Antagonist
- Reserve use for patients who have frequent flares and in whom first-line therapies are ineffective, contraindicated, or not tolerated
- 100 mg subq once daily until symptom improvement; usual duration: 3 to 5 days
- NOT routinely used in the ED.
Disposition
- Generally outpatient treatment
See Also
References
- ↑ Shah K, Spear J, Nathanson LA, Mccauley J, Edlow JA. Does the presence of crystal arthritis rule out septic arthritis?. J Emerg Med. 2007;32(1):23-6.
- ↑ Khanna D. et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. Oct 2012;64(10):1447-61
- ↑ Terkeltaub RA et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060.
- ↑ GlobalRPH. http://www.globalrph.com/gout.htm*colchicine
- ↑ Groff GD et al. Systemic steroid therapy for acute gout: a clinical trial and review of the literature. Semin Arthritis Rheum. 1990;19(6):329
- ↑ Janssens H. et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854.